• Robert J. Hawkins, B.Sc., DDS •
Background
Approximately 3,000 new cases of oral cancer are diagnosed each year in Canada. Most of
these cases occur among older adults with a history of tobacco use or excessive alcohol
consumption. Preventive interventions for oral cancer include counselling of patients to
modify risk factors and screening to identify precancerous and early-stage lesions. This
report presents evidence-based guidelines on the prevention of oral cancer and precancer
among asymptomatic patients.
Methods
Literature searches of the 1966-1999 MEDLINE and CANCERLIT databases were completed using
the major MeSH heading “mouth neoplasms”. References from articles and
recommendations of organizations were also reviewed. The evidence-based methods of the
Canadian Task Force on Preventive Health Care were used to assess evidence and to develop
guidelines. Advice from experts and other recommendations were taken into consideration.
Results
In cohort and case-control studies, smoking cessation decreased the risk of oral cancer
and precancer. Randomized controlled trials (RCTs) indicate counselling by trained health
care professionals is effective in promoting smoking cessation. Although counselling has
been effective for the reduction of excessive alcohol consumption in RCTs, no studies have
examined whether alcohol reduction reduces the risk of oral cancer or precancer. The
usefulness of general population screening is limited by the low prevalence and incidence
of the disease, the potential for false-positive diagnoses and the poor compliance with
screening and referral. There is no evidence that screening of the general population or
high-risk groups leads to a reduction in mortality or morbidity from oral cancer.
Interpretation
There is good evidence to specifically consider smoking cessation counselling in a
periodic health examination (grade A recommendation). For population screening, there is
fair evidence to specifically exclude screening for oral cancer (grade D recommendation).
For opportunistic screening during periodic examinations, there is insufficient evidence
to recommend inclusion or exclusion of screening for oral cancer (grade C recommendation).
For patients at high risk, annual examination by physician or dentist should be
considered. Risk factors include tobacco use and excessive consumption of alcohol. These
recommendations are similar to those made by the Canadian Task Force on the Periodic
Health Examination in 1994 and by the U.S. Preventive Services Task Force in 1996.
MeSH Key Words: counselling; mouth neoplasms; oral cancer, prevention; screening.
© J Can Dent Assoc 1999; 65:617
[Burden of Suffering Methods Results Interpretation Research Agenda Members of the Canadian Task Force on Preventive Health Care References]
Oral cancer accounts for about 3% to 4% of all cancers and 2% to 3% of cancer-related deaths.1 More than 90% of cases are squamous cell carcinomas, with the tongue and floor of mouth being the most common sites (75-85%). Despite low disease prevalences in developed countries, survival rates for patients with advanced stage lesions are generally 50% or less.2 A preclinical phase is detectable as a white or red lesion, and treatment at an early stage may improve survival rates to above 80%.3 Unfortunately, most patients (67-77%) do not seek consultation until advanced cancer is present with symptoms of persistent pain.4
Preventive interventions for oral cancer include counselling of patients to modify risk factors (e.g., tobacco use and excessive alcohol consumption) and screening to identify precancerous and early-stage lesions. Previous guidelines have recommended that health care professionals deliver smoking cessation counselling to patients,5,6 but uncertainty exists about the effectiveness of screening interventions. The use of a visual clinical examination to screen asymptomatic individuals for oral cancer and precancer has been advocated as an easy, non-invasive method to reduce disease-related morbidity and mortality,2 and the disease appears to fulfil many of the criteria for suitability for screening interventions.7 However, in North America, screening for oral cancer has been controversial because of the low prevalence and incidence of disease; the approximate number of new cases per year is 3,000 in Canada and 30,000 in the United States.8,9 Consequently, large numbers of people must be screened to identify the few who will benefit, and the lives of those saved must be weighed against the financial costs of screening and of incorrect diagnoses.
Burden of Suffering
The estimated incidence of oral cancer in Canada was 3,090 in 1996, and the
estimated number of deaths was 1,070 — 1.7% of all cancer deaths.8 From
1987 to 1991, the actual number of new cases per year showed little variation, ranging
from 2,837 to 3,017. The number of deaths during this period ranged from 960 to 1,026. The
potential years of life lost due to oral cancer was 17,000 in 1993. Most new cases were
found among men aged 50 and over (71% of all cases); for men, the probability of
developing oral cancer was found to increase from age 50 to 90 from 0.2% to 1.7%.
Reported five-year survival rates for patients are often 50% or lower. These rates have not improved substantially since the 1960s, because diagnosis usually occurs when nodal involvement and metastases have occurred (stages III or IV).9 In the advanced stages of the disease, morbidity and mortality are both high, and treatment at later stages may lead to impaired function, pain and disfigurement.10,11 Speech, appearance and chewing ability may all be adversely affected by the disease or its therapy. In a one-year follow-up study of patients who had received cancer therapy, side effects were found to affect eating in 23 of 25 patients.12 Financial costs of the disease are also high, since rehabilitation and prosthetic replacements are often necessary following treatment.
Extraction of Evidence
Inclusion and exclusion criteria were used to select appropriate studies. Case reports, expert opinions, review articles and abstracts were excluded. Studies of precancer and cancer therapies were included only if lesions were of the oral cavity or oropharynx: sites 140 to 149 of the International Classification of Diseases (ICD-9).13 Articles about cancer therapy had to include patients with early-stage disease. Specific outcome results for stage I or stage II had to be reported. (Stage I refers to the TNM classification T1N0M0 and stage II to T2N0M0; in both of these stages no nodal involvement or metastases are present.)
Critical Appraisal and Consensus Development
This evidence was systematically reviewed using the methodology of the
Canadian Task Force on Preventive Health Care. This Task Force of expert clinicians and
methodologists from a variety of medical specialities used a standardized, evidence-based
method for evaluating the effectiveness of this intervention. The lead author prepared a
manuscript providing critical appraisal of the evidence. This included identification and
critical appraisal of key studies and ratings of the quality of this evidence using the
Task Force’s established methodological hierarchy (Appendix 1), resulting in a
summary of proposed conclusions and recommendations for consideration by the Task Force.
This manuscript was precirculated to the members in April 1998, and evidence for this
topic was presented by the lead author and deliberated upon in a Task Force meeting in May
1998.
At the meeting, the expert panellists addressed critical issues, clarified ambiguous concepts and analysed the synthesis of the evidence. At the end of this process, the specific clinical recommendations proposed by the lead author were discussed, as were issues related to clarification of the recommendations for clinical application and any gaps in evidence. The results of this process are reflected in the description of the decision criteria presented with the specific recommendations. The final decisions on recommendations were arrived at unanimously by the group and the lead author.
Procedures to achieve adequate documentation, consistency, comprehensiveness, objectivity and adherence to the Task Force methodology were maintained at all stages during review development, the consensus process and beyond. These procedures were managed by the Task Force Office under supervision of the Chairman and ensured uniformity and impartiality throughout the review process. The full methodology is described in Woolf and others.14
Risk Factors
Although an interaction has been shown, the independent effects of tobacco and alcohol have been difficult to determine, and studies have found conflicting results. Both tobacco22,23 and alcohol18,24,25 have been described as the more important risk factor, while other researchers have found comparable results for the two factors or sex differences.15,16,26 In addition, two studies have found differences by anatomic location, alcohol being the stronger risk factor for oral and pharyngeal cancer and smoking the stronger factor for laryngeal cancer.18,20 A major difficulty in the study of tobacco and alcohol as risk factors is that most oral cancer patients have used both products. Further research is necessary to determine the relationship between oral cancer, alcohol use and tobacco use.
Other risk factors for oral cancer include previous upper aerodigestive tract malignancy or oral malignancy,27,28 an age of 60 or older,29 human papillomavirus30 and exposure to ultraviolet light (lip cancer).31 There is no known association between oral cancer and denture wearing or denture biomaterials.32-34 Betel-quid chewing has been shown to be associated with oral cancer in epidemiological studies.35,36 It is questionable, however, whether betel juice alone enhances the risk of oral cancer or if the effect is due to the tobacco added to the chewing mixture.36-38
Manoeuvres
Counselling to modify risk factors. Primary prevention of oral cancer may
involve counselling on the cessation of tobacco use or counselling on the reduction of
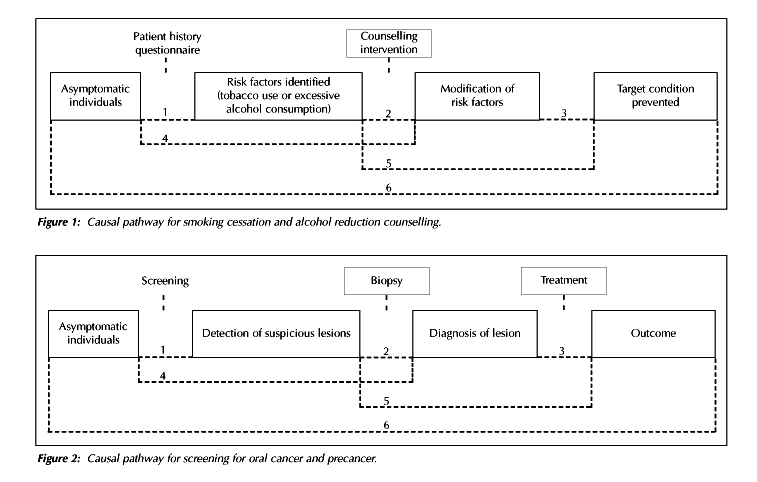
alcohol consumption. Figure 1 shows the causal pathway for the counselling
manoeuvre.

Evidence from randomized controlled trials documents the effectiveness of smoking cessation counselling directed toward adults.39,40 Counselling by health care providers has been shown to increase smoking cessation rates over 6 to 12 months relative to interventions where there is no provider.39-42 Health care providers in these studies include physicians, dentists, nurses and dental hygienists. However, a lack of training or interest in smoking cessation exists among many health care professionals, especially dentists. Physicians are more likely than dentists to report that they routinely advise smoking patients to quit; 30% to 40% of dentists and 70% to 80% of physicians report doing smoking cessation counselling.43 Results from a population-based survey of dental patients also suggest that dentists under-utilize tobacco cessation advice.44
In randomized controlled trials, counselling for the reduction of excessive alcohol consumption, defined as 15 or more drinks per week,15 has been found to significantly reduce alcohol consumption among problem drinkers and to reduce the frequency of binge drinking and excessive drinking.45-48 A limitation of these studies is the use of self-report data, although several studies also conducted family-member interviews to corroborate subject self-report. Counselling for alcohol reduction has been assessed only among medical professionals. Dentists should refer patients with alcohol problems to a trained medical professional.
Screening for oral cancer and precancer. Figure 2 shows the causal pathway for the screening manoeuvre. The assessment of screening interventions examines (a) the ability of examiners to identify suspicious lesions and (b) the accuracy of diagnostic procedures.
(a) Population-based studies of screening programs to identify suspicious lesions by oral physical examination have generally found high specificity (98-99%), but sensitivity has varied greatly (56-94%) (Table 1).49-53 Positive predictive values have also varied (15-91%) depending on the prevalence of oral cancer. Consequently, due to the low prevalence of oral cancer in developed countries, two significant issues for screening programs are a low yield in the general population and a high proportion of false-positive referrals
Table 1 Results from studies of screening for oral cancer by clinical examination
| Study | N |
Examiners | Gold Standard | Sensitivity | Specificity | Type of Program |
Mehta and others49 |
33,331 | 35 trained | 2 dentists | 56% | 98% | Regional screening health workers program in India |
| keda and others50 | 3,131 | 4 general dentists | 1 oral pathologist | 73% | 73% | Screening program for factory and office workers in Japan |
| Downer and others51 | 553 | 2 general dentists | 1 specialist in oral medicine | 71% | 99% | Company screening program in London (UK) |
| Jullien and others52 | 985 | 2 general dentists | 1 specialist in oral medicine | 74% | 99% | Screening in UK dental hospital and medical practice |
| Mathew and others53 | 2,069 | 42 trained health workers | 3 physicians | 94% | 99% | Regional screening program in India |
.
In eight reports of population-based screening efforts, the yield of suspicious lesions was less than 6% overall and was under 2% in five studies (Table 2).49-52,54-57 The yield of cancerous lesions, confirmed by biopsy, was much lower (not greater than 0.05%), and three studies failed to detect any cancerous lesions. Screening programs that focus on high-risk groups may substantially increase the yield for both suspicious and confirmed lesions. A screening program in northern Italy for older male alcoholics reported that 5 of 212 subjects had oral cancer, a yield of 2.4%.58
Table 2 Yield of suspicious and cancerous lesions from studies of screening for oral cancer by clinical examination
| Study | N | Site | Yield of Suspicious Lesions (%) | Yield of Confirmed Cancerous Lesions (%) | Number of Confirmed Cancerous Lesions |
| Bouquot and Gorlin54 | 23,616 | USA | 3.4 | 0.01 | 2 |
| Mehta and others49 | 33,331 | India | 1.3 | 0.05 | 20 |
| Banoczy and others55 | 7,820 | Hungary | 1.3 | 0.05 | 1 |
| Ikeda and others50 | 3,131 | Japan | 5.3 | 0.0 | 0 |
| Talamini and others58 | 212 | Italy - high risk group | 9.5 | 2.4 | 5 |
| Downer and others51 | 553 | UK | 5.5 | 0.0 | 00 |
| Fernandez and others56 | 13 million | Cuba | 0.2 | 0.005 | 705 |
| Field and others57 | 1,949 | UK | 0.2 | 0.05 | 1 |
| Jullien and others52 | 985 | UK | 1.2 | 0.0 | 0 |
The above studies must be interpreted with caution because of the use of specialists as the gold standard for evaluation and the use of different types of health workers as examiners. Other variations between studies include the protocol for training examiners, the criteria used for diagnosing lesions, the location where examinations were performed and the equipment and light source used. Not surprisingly, the highest diagnostic values (94% and 99%) were obtained in the study that used the most extensive training program.53 In this study, examiners underwent a 6-week program of lectures and clinical education in epidemiology, diagnosis and the management and prevention of oral precancer and cancer; the examiners were then tested (written and practical). Based on the test results, the best health workers were selected as examiners. In other studies, training programs were not as extensive and were either of 2 to 5 days49 or of an unspecified duration.50-52
The three studies employing dentists as examiners had similar results for sensitivity (71-74%).50-52 These values were low compared to a sensitivity of 94% obtained in a study using other health care workers.53 A possible explanation may be that dentists in these studies were not formally trained in standardization, and were only advised of the diagnostic criteria for identifying positive and negative cases. The low sensitivity results may reflect general dentists’ lack of training in the detection of oral cancer and precancer and a failure to seek continuing education to maintain their training. Studies to determine the sensitivity and specificity of oral cancer examinations conducted by physicians have not yet been done.
Vital staining of suspected lesions with toluidine blue (tolonium chloride) might serve as an adjunct to visual examination. Sensitivity and specificity are high (average 97% and 91%) when staining is done by experienced clinicians in specialized institutions, but the test characteristics are unknown for less experienced clinicians in general practice settings.59,60 For screening purposes, results from a meta-analysis suggest that vital staining is of limited usefulness due to the low prevalence of oral cancer.59 The yield of suspicious lesions would be increased, but this increase would not be substantial.
Regardless of their response to toluidine blue staining, all suspicious lesions should undergo tissue biopsy. Staining is not a substitute for biopsy nor is it a replacement for detailed visual and digital head and neck examination. However, tissue stains may assist clinicians in determining the extent of lesions, in selecting sites for biopsy and in following up patients after cancer treatment.61 Therefore, the use of vital staining as a screening measure in the general population is not supported, but this procedure may be useful in the assessment of high-risk patients and suspicious lesions.
(b) Screening by clinical examination is not intended to be diagnostic. Biopsy is currently recognized as the definitive method of diagnosing oral cancer. However, recent studies of observer agreement have led researchers to question the ability of oral pathologists to diagnose cases based on histologic examination. Three studies (one in Denmark and two in the United States) have assessed inter-rater agreement between oral pathologists in the diagnosis of oral epithelial dysplasia (one of the criteria for malignant diagnosis).62-64 All studies involved the examination of histologic specimens by pathologists followed by a comparison with either other pathologists or the sign-out diagnosis. The total number of slides examined by each observer ranged from 100 to 120; slides represented a spectrum of dysplasia, which varied, in the authors’ opinions, from no dysplasia to severe dysplasia, or carcinoma-in-situ. Observers classified dysplasia according to the following grades: none, mild, moderate and severe. Kappa values for exact agreement of diagnosis did not exceed moderate agreement and ranged from 0.15 to 0.45.62,63 The addition of clinical information did not improve agreement; it actually decreased agreement (0.10-0.23).64 For agreement within one diagnostic grade, agreement was much higher, ranging from 0.70 to 0.88.63 Disagreements of two or more grades occurred in 6% to 20% of cases.
Most classification differences were of one grade; in those cases, it is likely that treatment planning decisions would not have been substantially different. However, no studies have examined the influence of biopsy assessment on treatment planning, and other factors must also be considered (e.g., patient history). Thus, the clinical significance of inter-examiner differences can only be speculative. Nevertheless, these results indicate that classification of dysplasia is an inexact science. Further advances in molecular biology may provide more objectivity and consistency in the assessment of lesions and their prognosis.65
Effectiveness of Counselling and Screening
Effectiveness of counselling. Case-control studies indicate that smoking
cessation decreases the risk of developing oral cancer.15,23,66 Odds ratios for
ex-smokers become equal to ratios for non-smokers after 10 years of cessation.
Furthermore, a 10-year follow-up study in India found that an anti-tobacco education
program resulted in a decrease in the incidence of precancerous lesions among the
intervention cohort compared to a control group.67 However, school-based
programs have had mixed results,68-70 and smokeless tobacco cessation programs
have been assessed only in small case series.71
Studies have failed to show that alcohol reduction leads to a decrease in the risk of oral cancer or precancer. Further research is necessary to establish the link between alcohol reduction and end-state outcomes (i.e., causal links 3, 5 or 6).
Effectiveness of screening. At present, only one study has reported data applicable to “causal link 6”, which represents the most direct line of evidence. In a non-controlled study of an oral cancer screening program in Cuba, approximately 13 million examinations were performed in government-sponsored dental offices over a six-year period (1984-1990).56 Although the proportion of early stage cancers detected by examiners increased from 24% to 49%, there was no change in Cuba’s oral cancer incidence and mortality over the study period. The program identified only 16% of the new oral cancer cases reported by the cancer registry during this period. However, the usefulness of these results is limited due to problems in study design: no control group was used, and the time period may have been too brief to detect improvements. It is doubtful that the screening program was implemented as intended, because less than 30% of subjects with suspected lesions complied with referral and less than one-quarter of the target population was screened annually.
No randomized controlled studies have yet evaluated the effectiveness of screening for oral cancer. In 1995, a randomized controlled screening intervention study was begun in India, but results will not be available for at least 7 to 10 years.53 When available, these results should be interpreted with caution, because of their lack of generalizability to Canada. The oral cavity is the leading site of cancer in India, and the use of population-based screening programs is more feasible. The results may, however, be applicable to certain high-risk subgroups within Canada (e.g., people from Southeast Asia).
Effectiveness of Treatment
Oral precancerous lesions. Oral precancer refers to lesions considered to
have malignant potential because they may exhibit dysplasia. Oral epithelial dysplasia may
present clinically as leukoplakia, erythroplakia or leukoerythoplakia. Prevalence of these
lesions is quite low (1-4%),54,55,72,73 and malignant transformation rates vary
from 0.1% to 6% in general population studies74-76 to 7% to 36% among high-risk
patients.77-80 Erythroplakia is considered to be an early sign of oral cancer,
because lesions that are erythroplastic or leukoerythroplastic have a higher risk of
malignant transformation compared to leukoplastic lesions79 and because the
majority of invasive cancers are red or predominantly red (64-86%).81,82
Another type of premalignant lesion, lichen planus, also has low prevalence (0.1-2%)54,55,83
and low transformation rates (0.4-3%).84-87 At present, it is not possible to
predict which precancerous lesions will ultimately undergo malignant transformation.
For localized lesions, surgical removal is the standard therapy, but its effectiveness has not been evaluated in randomized controlled studies. Observational studies have found that, after therapy, the recurrence rate of premalignant lesions is approximately 20% and the risk of developing malignant lesions is not eliminated (5%).88-90 The number of lesions prevented from malignant development by surgical excision cannot be determined from these studies. Another mode of therapy, laser removal, has yet to be assessed in well-designed clinical trials.91
For certain lesions, surgical excision may be difficult, because of the location or extent of the lesion. Three therapies have been assessed in clinical trials for such lesions: 13-cis-retinoic acid (13cRA),92,93 beta-carotene (a retinol precursor)94 and bleomycin.95 In a randomized controlled trial, high doses of 13cRA were more effective than a placebo in reversing and stabilizing oral leukoplakia; however, side effects and relapse after discontinuation were significant problems.92 A recent comparison study of low-dose regimens of 13cRA indicated that 13cRA was more effective than beta-carotene; only 8% of cases progressed to malignancy, compared to 55% in the beta-carotene group.93 Side effects were more common for the 13cRA group, but only mild complications were reported. A third agent, topical bleomycin, was more effective than a placebo in decreasing lesion size in a randomized trial of patients with oral leukoplakia.95 No studies have reported a comparison of 13cRA and bleomycin therapy.
Early stage malignant lesions. Malignant lesions identified through screening examinations are usually at an early stage (I or II), the tongue and floor of the mouth being the most common sites. No controlled studies have yet evaluated either surgery or radiotherapy. Since 1980, nine studies have reported data from retrospective reviews of patient charts (Table 3).96-104 The only measure provided in all studies was the five-year survival rate; for stage I, five-year survival ranged from 57% to 90%, and for stage II, from 41% to 72%. A problem with the statistical analysis of these data is that the influence of lead-time bias was not considered.
Table 3 Results from studies* of therapy for early stage oral cancer (stages I and II)
| Study | N | Intervention | Site | Outcome (5-yearsurvival rate) |
Decroix and Ghossein96 |
382 | Radiotherapy or combination |
Tongue | Stage I - 57% Stage II - 41% |
Callery and others97 |
546 | Surgical
|
Tongue | Stage I - 65%
Stage II - 58% |
Mendenhall and others98 |
132 | Radiotherapy |
Tongue | Stage II - 54% |
Nason and others99 |
209 | Surgical |
Floor of mouth | Stage I - 69%
Stage II - 64% |
Wildt and others100 |
267 | Surgery(40%) Radiotherapy(40%) Combined (22%) |
Various sites: primarily mandibular | Stage I - 65% Stage II - 42% |
| Soderholm101 | 162 | Surgery(20%) Radiotherapy(18%) Combined (62%) |
Mandibular region | Stage I - 80% Stage II - 58% |
Franceschi and others102 |
297 | Surgical |
Tongue | Stage I - 90% Stage II - 72% |
| Kraus and others103 | 100 | Surgical | Tongue | Stage I - 1/11- 77% |
| Lefebvre and other104 | 429 | Radiotherapy | Various sites: primarily tongue and floor of mouth | Stage I - 61% Stage II -50% |
A valid comparison of surgery and radiotherapy is difficult because of the poor quality of the studies and the inability to adjust for patient differences between studies. Since definitive conclusions can be drawn only from randomized controlled trials, survival rates at present can only be described as comparable for surgery and radiotherapy. Another problem in attempting to evaluate cancer therapy is that recurrence rates for specific cancer stages are not reported in most studies. Only two studies have documented recurrence rates at five years for stage I (12-14%) and stage II (18-22%).96,104
Finally, few studies provide information on the relative impact of therapy on quality of life and oral function. Length of survival alone is an unsatisfactory measure of the success of treatment; the quality of survival needs to be evaluated as well as the quantity. At present, subjective measures of outcome have been used mainly in studies of advanced cancer therapy.10-12 No studies have compared the health states achieved through therapy to the health states of people who refused treatment.
Canadian Task Force Recommendations
Table 4 summarizes the recommendations developed from this review. There is
good evidence to support the recommendation that counselling for smoking cessation should
be specifically considered in a periodic health examination (A-level recommendation). No
specific recommendation was made for alcohol reduction counselling for the prevention of
oral cancer; however, counselling of problem drinkers may be recommended for other
reasons. The Task Force gave counselling a B recommendation in 1994.105
Table 4 Summary table of recommendations for prevention of oral cancer mortality
| Manoeuvre | Effectiveness | Level of Evidence | Recommendation |
| Smoking cessation counselling |
Multiple interventions and reinforcement strategies have increased 6-month and 1-year cessation rates. Smoking
cessation has been shown to reduce the risk of oral cancer. Intervention programs have
reduced the incidence of precancerous lesions. |
Randomized controlled trials39,40 (I)
Case-control and cohort |
Good evidence to specifically consider smoking
cessation counselling in a PHE (A) Counselling should be done by trained health professionals. |
| Screening by clinical examination |
The usefulness of screening is limited by: the
low prevalence and incidence of disease, the potential for false positive diagnoses and
the poor compliance with screening and referral. No studies have shown that screening
intervention programs reduce mortality or morbidity due to oral cancer. |
Case-control studies49-58 (II-2) |
Population screening: Fair
evidence to exclude screening the general population for oral cancer by clinical
examination (D) Opportunistic screening: Insufficient evidence to recommend inclusion
or exclusion of screening for oral cancer by clinical examination in a PHE of asymptomatic
patients (C) |
Recommendations for screening are divided into two components: population screening and opportunistic screening (i.e., screening during periodic examinations). For population screening, a D recommendation was made based on the low prevalence and incidence of oral cancer in Canada, the low yields obtained in screening studies and the potential for high proportions of false positive diagnoses. False positives are not an insignificant problem, because they may lead to the personal and financial costs of anxiety, unnecessary biopsies and inappropriate therapy.
For opportunistic screening of asymptomatic patients, a C recommendation is made, similar to the recommendation made by the Task Force in 1994.5 For patients at high risk, an annual examination by a physician or dentist should be considered. Tobacco use and excessive alcohol consumption, alone or in combination, are the most important factors linked to the development of oral cancer.
Recommendations of Others
The recommendation for smoking cessation counselling agrees with the
guidelines developed by the U.S. Department of Health and Human Services (1996).6
The screening recommendations in this report are consistent with recommendations from the
U.S. Preventive Services Task Force and the U.K. Working Group on Oral Cancer.106-108
Both have indicated screening only for high-risk groups. Conversely, routine screening for
asymptomatic persons over 20 years of age was advocated by the American Cancer Society.109
Dental organizations have also supported the concept of oral cancer screening, but no
official statements have been made.110,111
Quality of evidence continues to be a major concern in the evaluation of oral cancer screening. No controlled prospective trials have yet linked screening to lives saved from oral cancer. As with screening for other forms of cancer, “the problem ... is not evidence of a lack of effect, but lack of evidence”.112
Research Agenda
1. In Canada, a national screening program is unlikely to be a
practical means of screening. However, the prospective evaluation of screening programs
for high-risk groups is warranted, as risk factors are known and identifiable. High-risk
individuals may be selected in a two-stage screening process consisting of a
self-administered questionnaire to identify patients with risk factors and a subsequent
oral cancer examination for those individuals classified as high-risk. The screening only
of high-risk groups would likely increase the yield of screening programs and may be more
cost-effective.113,114 However, one study that screened older male alcoholics
suggests that it is expensive to identify high-risk individuals and that compliance with
referral is poor (34%).58 Therefore, cost-effective ways of identifying these
individuals and effective follow-up programs are necessary.
2. Another issue needing further consideration is which health care professionals should perform screening examinations and counselling interventions. In a study of case referrals, physicians identified a higher proportion of cancers located in the pharynx, larynx and tonsil, whereas dentists identified a higher percentage of cases in the gingiva and floor of the mouth.115 Dentists were also more likely to identify cases in the earlier, asymptomatic stages of cancer and precancer, while symptomatic patients generally reported to a physician for examination.
Both general dentists and physicians are able to detect cancerous or precancerous lesions in their practices, but it is unknown whether one profession is more suitable. Although dentists conduct oral examinations more often and may be expected to be more familiar with the differences between pathology and variations of the normal, studies using general dentists as screening examiners have found sensitivity values to range from 71% to 74%,50-52 indicating a high rate of false negatives. Primary care practitioners have also been found to have difficulties identifying oral lesions.116 The lack of training and awareness among practitioners in medicine and dentistry has been noted.117-119
It has been suggested that a variety of health professions should play a role in counselling and screening and that emphasis should be placed on level of training and interest rather than on membership in a specific professional discipline.6 From a cost-effectiveness viewpoint, the use of nurses, nurse practitioners and dental hygienists would be substantially less expensive.
3. Further studies are necessary to establish a causal link between alcohol reduction and reduced risk of oral cancer and precancer. A case-control study would be the most feasible means of examining this risk factor, but a sufficient number of subjects are needed to control for the effects of tobacco use.
Members of the
Canadian Task Force on Preventive Health Care
Chairman: Dr. John W. Feightner, professor, department of family
medicine, University of Western Ontario, London, Ont. Past chairman: Dr. Richard
Goldbloom, professor, department of pediatrics, Dalhousie University, Halifax. Members:
Drs. R. Wayne Elford, professor emeritus, department of family medicine, University of
Calgary, Calgary; Michel Labrecque, associate professor and director of research,
department of family medicine and Centre Hospitalier Universitaire de Québec, Laval
University, Quebec; Harriet MacMillan, associate professor, departments of psychiatry
andpediatrics and Centre for Studies of Children at Risk, McMaster University, Hamilton,
Ont.; Robin McLeod, professor, department of surgery, Mount Sinai Hospital and University
of Toronto, Toronto; Jean-Marie Moutquin, professor, department of obstetrics and
gynecology and Centre de recherche de l’Hôpital Saint-François d’Assise du
CHUQ, Laval University, Quebec; Christopher Patterson, professor and head, division of
geriatric medicine, department of medicine, McMaster University, Hamilton, Ont.; Elaine
E.L. Wang, associate professor, departments of pediatrics and public health sciences,
faculty of medicine, University of Toronto, Toronto. Resource people: Nadine
Wathen, coordinator, Canadian Task Force on Preventive Health Care, department of family
medicine, University of Western Ontario, London, Ont., and Tim Pauley, research assistant,
Canadian Task Force on Preventive Health Care, department of family medicine, University
of Western Ontario, London, Ont.
Acknowledgments: We thank Dr. Joel B. Epstein, British Columbia Cancer Agency, Vancouver, and Dr. Thomas D. Daley, department of oral pathology, University of Western Ontario, London, Ont., for reviewing a draft form of this report. The views expressed in this report are those of the author and the Task Force and do not necessarily reflect the positions of reviewers.
Dr. Hawkins is a graduate student in community dentistry, faculty of dentistry, University of Toronto.
Dr. Wang is associate professor, departments of pediatrics and public health sciences, faculty of medicine, University of Toronto.
Dr. Leake is professor of community dentistry, faculty of dentistry, University of Toronto.
The views expressed are those of the author and do not necessarily reflect the opinion or official policies of the Canadian Dental Association.
Appendix 1
Canadian Task Force on Preventive Health Care: Levels of Evidence and Grades of Recommendations
| Quality of Published Evidence | |
| I | Evidence from at least 1 properly randomized controlled trial (RCT). |
| II-1 | Evidence from well-designed controlled trials without randomization. |
| II-2 | Evidence from well-designed cohort or case-control analytic studies, preferably from more than 1 centre or research group. |
| II-3 | Evidence from comparisons between times or places with or without the intervention. |
| III | Opinions of respected authorities based on clinical experience, descriptive studies or reports of expert committees. |
| Grades of Recommendations | |
| A | Good evidence to support the recommendation that the condition be specifically considered in a periodic health examination (PHE). |
| B | Fair evidence to support the recommendation that the condition be specifically considered in a PHE. |
| C | Insufficient evidence regarding inclusion or exclusion of the condition in a PHE, but recommendations may be made on other grounds. |
| D | Fair evidence to support the recommendation that the condition be specifically excluded in a PHE. |
| E | Good evidence to support the recommendation that the condition be specifically excluded in a PHE. |
1. Parkin DM, Laara E, Muir CS. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer 1988; 41:184- 97.
2. Silverman S Jr. Early diagnosis of oral cancer. Cancer 1988; 62:1796-9.
3. Speight PM, Morgan PR. The natural history and pathology of oral cancer and precancer. Community Dent Health 1993; 10(Suppl 1):31-41.
4. Guggenheimer J, Verbin RS, Johnson JT, Harkowitz CA, Myers EN. Factors delaying the diagnosis of oral and oropharyngeal carcinomas. Cancer 1989; 64:932-5.
5. Rosati C. Prevention of Oral Cancer. In: The Canadian guide to clinical practice health care. The Canadian Task Force on the Periodic Health Examination. Ottawa: Minister of Supply and Services, 1994.
6. Fiore MC, Bailey WC, Cohen SJ, and others. Smoking Cessation. Clinical Practice Guideline No. 18. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Publication No. 96-0692. April 1996.
7. Hill GB, Spitzer WO, Ferenzi LZ. Optimal frequency of examinations aimed at detecting pre-symptomatic disease. Can J Public Health 1984; 75:419-24.
8. National Cancer Institute of Canada. Canadian Cancer Statistics 1996. Toronto, Canada, 1996.
9. Wingo PA, Tong T, Bolden S. Cancer statistics, 1995 [published erratum appears in CA Cancer J Clin 1995; 45:127-8]. CA Cancer J Clin 1995; 45:8-30.
10. Bundgaard T, Tandrup O, Elbrond O. A functional evaluation of patients treated for oral cancer. A prospective study. Int J Oral Maxillofac Surg 1993; 22:28-34.
11. Rathmell AJ, Ash DV, Howes M, Nicholls J. Assessing quality of life in patients treated for advanced head and neck cancer. Clin Oncol (R Coll Radiol) 1991; 3:10-6.
12. Beeken L, Calman F. A return to “normal eating” after curative treatment for oral cancer. What are the long-term prospects? Eur J Cancer B Oral Oncol 1994; 30B:387-92.
13. World Health Organization. Application of the international classification of diseases to dentistry and stomatology. Geneva: WHO, 1978.
14. Woolf SH, Battista RN, Anderson GM, Logan AG, Wang E. Assessing the clinical effectiveness of preventive maneuvers: analytic principles and systematic methods in reviewing evidence and developing clinical practice recommendations. A report by the Canadian Task Force on the Periodic Health Examination. J Clin Epidemiol 1990; 43:891-905.
15. Blot WJ, McLaughlin JK, Winn DM, Austin DE, Greenberg RS, Preston-Martin S, and others. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988; 48:3282-7.
16. Rothman K, Keller A. The effect of joint exposure to alcohol and tobacco on the risk of cancer of the mouth and pharynx. J Chronic Dis 1972; 25:711-6.
17. Schmidt W, Popham RE. The role of drinking and smoking in mortality from cancer and other causes in male alcoholics. Cancer 1981; 47:1031-41.
18. Elwood JM, Pearson JC, Skippen DH, Jackson SM. Alcohol, smoking, social and occupational factors in the aetiology of cancer of the oral cavity, pharynx and larynx. Int J Cancer 1984; 34:603-12.
19. Zheng TZ, Boyle P, Hu HF, Duan J, Jiang PJ, Ma DQ, and others. Tobacco smoking, alcohol consumption and risk of oral cancer: a case-control study in Beijing, People’s Republic of China. Cancer Causes Control 1990; 1:173-9.
20. Choi SY, Kahyo H. Effect of cigarette smoking and alcohol consumption in the etiology of cancer of the oral cavity. Int J Epidemiol 1991; 20:878-85.
21. Kato I, Nomura AM, Stemmermann GN, Chyou PH. Prospective study of the association of alcohol with cancer of the upper aerodigestive tract and other sites. Cancer Causes Control 1992; 3:145-51.
22. Graham S, Dayal H, Rohrer J, Swanson M, Shultz H, Shedd D, and others. Dentition, diet, tobacco, and alcohol in the epidemiology of oral cancer. J Natl Cancer Inst 1977; 59:1611-7.
23. Macfarlane GJ, Zheng TZ, Marshall JR, Boffetta P, Niu S, Brasure J and others. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur J Cancer B Oral Oncol 1995; 31B:181-7.
24. Mashberg A, Garfinkel L, Harris S. Alcohol as a primary risk factor in oral squamous carcinoma. CA Cancer J Clin 1981; 31:146- 56.
25. Brugere J, Guenel P, Leclerc A, Rodriguez J. Differential effects of tobacco and alcohol in cancer of the larynx, pharynx, and mouth. Cancer 1986; 57:391-5.
26. Wynder EL, Stellman SD. Comparative epidemiology of tobacco-related cancers. Cancer Res 1977; 37:4608-22.
27. Franco EL, Kowalski LP, Kanda JL. Risk factors for second cancers of the upper respiratory and digestive systems: a case-control study. J Clin Epidemiol 1991; 44:615-25.
28. Jovanovic A, van der Tol IG, Schulten EA, Kortense PJ, de Vries N, Snow GB, and others. Risk of multiple primary tumors following oral squamous-cell carcinoma. Int J Cancer 1994; 56:320-3.
29. Boyle P, Macfarlane GJ, Maisonneuve P, Zheng T, Scully C, Tedesco B. Epidemiology of mouth cancer in 1989; a review. J R Soc Med 1990; 83:724-30.
30. International Agency for Research in Cancer Monographs. Studies in Cancer in Humans 1995; 64:189-92.
31. Spitz MR. Epidemiology and risk factors for head and neck cancer. Sem Oncol 1994; 21:281-8.
32. Gorsky M, Silverman S Jr. Denture wearing and oral cancer. J Prosthet Dent 1984; 52:164-6.
33. Zheng TZ, Boyle P, Hu HF, Duan J, Jian PJ, Ma DQ, and others. Dentition, oral hygiene, and risk of oral cancer: a case-control study in Beijing, People’s Republic of China. Cancer Causes Control 1990; 1:235-41.
34. Morse DE, Katz RV, Pendrys DG, Holford TR, Krutchkoff DJ, Eisenberg E, and others. Mouthwash use and dentures in relation to oral epithelial dysplasia. Oral Oncol 1997; 33:338-43.
35. World Health Organization. Control of oral cancer in developing countries. Bulletin of the World Health Organization 1984; 62:817-830.
36. Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai SC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med 1995; 24:450-3.
37. Gupta PC, Pindborg JJ, Mehta FS. Comparison of carcinogenicity of betel quid with and without tobacco: an epidemiological review. Ecol Dis 1982; 1:213-9.
38. Johnson NW, Warnakulasuriya KA. Epidemiology and aetiology of oral cancer in the United Kingdom. Community Dent Health 1993; 10(Suppl 1):13-29.
39. Cohen SJ, Stookey GK, Katz BP, Drook CA, Smith DM. Encouraging primary care physicians to help smokers quit: a randomized controlled trial. Ann Intern Med 1989; 110:648- 52.
40. Cohen SJ, Stookey GK, Katz BP, Drook CA, Christen AG. Helping smokers quit: a randomized controlled trial with private practice dentists. J Am Dent Assoc 1989; 118:41-5.
41. Little SJ, Stevens VJ, Severson HH, Lichtenstein E. Effective smokeless tobacco intervention for dental hygiene patients. J Dent Hyg 1992; 66:185-90.
42. Centers for Disease Control. Physician and other health-care professional counseling of smokers to quit — United States, 1991. MMWR Morb Mortal Wkly Rep 1993; 42:854-7.
43. Secker-Walker RH, Solomon LJ, Flynn BS, Dana GS. Comparisons of the smoking cessation counseling activities of six types of health professionals. Prev Med 1994; 23:800-8.
44. Martin LM, Bouquot JE, Wingo PA, Heath CW Jr. Cancer prevention in the dental practice: oral cancer screening and tobacco cessation advice. J Public Health Dent 1996; 56:336-40.
45. Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988; 297:663-8.
46. Kahan M, Wilson L, Becker L. Effectiveness of physician-based interventions with problem drinkers: a review. CMAJ 1995; 152:851-9.
47. A cross-national trial of brief interventions with heavy drinkers. World Health Organization Brief Intervention Study Group. Am J Public Health 1996; 86:948-55.
48. Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers. A randomized controlled trial in community-based primary care practices. JAMA 1997; 277:1039-45.
49. Mehta FS, Gupta PC, Bhonsle RB, Murti PR, Daftary DK, Pindborg JJ. Detection of oral cancer using basic health workers in an area of high oral cancer incidence in India. Cancer Detect Prev 1986; 9:219-25.
50. Ikeda N, Ishii T, Iida S, Kawai T. Epidemiological study of oral leukoplakia based on mass screening for oral mucosal diseases in a selected Japanese population. Community Dent Oral Epidemiol 1991; 19:160-3.
51. Downer MC, Evans AW, Hughes Hallett CM, Jullien JA, Speight PM, Zakrzewska JM. Evaluation of screening for oral cancer and precancer in a company headquarters. Community Dent Oral Epidemiol 1995; 23:84-8.
52. Jullien JA, Downer MC, Zakrzewska JM, Speight PM. Evaluation of a screening test for early detection of oral cancer and precancer. Community Dent Health 1995; 12:3-7.
53. Mathew B, Sankaranarayaan R, Sunilkumar KB, Kuvurila B, Pisani P, Nair MK. Reproducibility and validity of oral visual inspection by trained health workers in the detection of oral precancer and cancer. Br J Cancer 1997; 76:390-4.
54. Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol 1986; 61:373-81.
55. Banoczy J, Rigo O. Prevalence study of oral precancerous lesions within a complex screening system in Hungary. Community Dent Oral Epidemiol 1991; 19:265-7.
56. Frenandez Garrote L, Sankaranarayanan R, Lence Anta JJ, Rodriguez Salva A, Maxwell Parkin D. An evaluation of the oral cancer control program in Cuba. Epidemiol 1995; 6:428-31.
57. Field EA, Morrison T, Darling AE, Parr TA, Zakrzewska JM. Oral mucosal screening as an integral part of routine dental care. Br Dent J 1995; 179:262-6.
58. Talamini R, Barzan L, Franceschi S, Caruso G, Gasparin A, Comoretto R. Determinants of compliance with an early detection programme for cancer of the head and neck in north-eastern Italy. Eur J Cancer B Oral Oncol 1994; 30B:415-8.
59. Rosenberg D, Cretin S. Use of meta-analysis to evaluate tolonium chloride in oral cancer screening. Oral Surg Oral Med Oral Pathol 1989; 67:621-7.
60. Epstein JB, Scully C, Spinelli JJ. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med 1992; 21:160-3.
61. Epstein JB, Oakley C, Millner A, Emerton S, van der Meij E, Le N. The utility of toluidine blue application as a diagnostic aid in patients previously treated for upper oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997; 83:537-47.
62. Karabulut A, Reibel J, Therkildsen MH, Praetorius F, Nielsen HW, Dabelsteen E. Observer variability in the histologic assessment of oral premalignant lesions. J Oral Pathol Med 1995; 24:198-200.
63. Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG, Svirsky JA, and others. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 80:188-91.
64. Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG, Svirsky JA, and others. The effect of clinical information on the histopathologic diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 85:74-7.
65. Epstein JB, Scully C. Assessing the patient at risk for oral squamous cell carcinoma. Spec Care Dent 1997; 17:120-8.
66. Kabat GC, Chang CJ, Wynder EL. The role of tobacco, alcohol use, and body mass index in oral and pharyngeal cancer. Int J Epidemiol 1994; 23:1137-44.
67. Gupta PC, Mehta FS, Pindborg JJ, Bhonsle RB, Murti PR, Daftary DK, and others. Primary prevention trial of oral cancer in India: a 10-year follow-up study. J Oral Pathol Med 1992; 21:433-9.
68. Sussman S, Dent CW, Stacy AW, Sun P, Craig S, Simon TR, and others. Project towards no tobacco use: 1-year behaviour outcomes. Am J Public Health 1993; 83:1245-50.
69. Elder JP, Wildey M, de Moor C, Sallis JF Jr, Eckhardt L, Edwards C, and others. The long-term prevention of tobacco use among junior high school students: classroom and telephone interventions. Am J Public Health 1993; 83:1239-44.
70. Stevens MM, Freeman DH Jr, Mott LA, Youells FE, Linsey SC. Smokeless tobacco use among children: the New Hampshire study. Am J Prev Med 1993; 9:160-7.
71. U.S. Department of Health and Human Services, Public Health Service, National Institute of Health: Smoking and Tobacco Control Monograph 2. Smokeless tobacco or health: An international perspective. September 1992.
72. Axell T. Occurrence of leukoplakia and some other oral white lesions among 20,333 adult Swedish people. Community Dent Oral Epidemiol 1987; 15:46-51.
73. Hogewind WF, van der Waal I. Prevalence study of oral leukoplakia in a selected population of 1000 patients from the Netherlands. Community Dent Oral Epidemiol 1988; 16:302-5.
74. Malaovalla AM, Silverman S, Mani NJ, Bilimoria KF, Smith LW. Oral cancer in 57,518 industrial workers of Gujarat, India: A prevalence and follow-up study. Cancer 1976; 37:1882-6.
75. Banoczy J. Follow-up studies in oral leukoplakia. J Maxillofac Surg 1977; 5:69-75.
76. Gupta PC, Mehta FS, Daftary DK, Pindborg JJ, Bhonsle RB, Jalnawalla PN, and others. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10-year follow-up study of Indian villagers. Community Dent Oral Epidemiol 1980; 8:283-333.
77. Banoczy J, Csiba A. Occurrence of epithelial dysplasia in oral leukoplakia. Analysis and follow up study of 120 cases. Oral Surg Oral Med Oral Pathol 1976; 42:766-74.
78. Pindborg JJ, Daftary DK, Mehta FS. A follow-up study of sixty-one oral dysplastic precancerous lesions in Indian villagers. Oral Surg Oral Med Oral Pathol 1977; 43:383-90.
79. Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer 1984; 53:563-8.
80. Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79:321-9.
81. Mashberg A. Erythroplasia: the earliest sign of asymptomatic oral cancer. J Am Dent Assoc 1978; 96:615-20.
82. Mashberg A, Feldman LJ. Clinical criteria for identifying early oral and oropharyngeal carcinoma: erythroplasia revisited. Am J Surg 1988; 156:273-5.
83. Axell T, Rundquist L. Oral lichen planus — a demographic study. Community Dent Oral Epidemiol 1987; 15:52-6.
84. Fulling HJ. Cancer development in oral lichen planus. A follow-up study of 327 patients. Arch Dermatol 1973; 108:667-9.
85. Silverman S Jr, Griffith M. Studies on oral lichen planus. II. Follow-up on 200 patients, clinical characteristics, and associated malignancy. Oral Surg Oral Med Oral Pathol 1974; 37:705-10.
86. Silverman S Jr, Gorsky M, Lozada-Nur F. A prospective follow-up study of 570 patients with oral lichen planus: persistence, remission, and malignant association. Oral Surg Oral Med Oral Pathol 1985; 60:30-4.
87. Markopoulos AK, Antoniades D, Papanayotou P, Trigonidis G. Malignant potential of oral lichen planus: a follow-up study of 326 patients. Oral Oncol 1997; 33:263-9.
88. Mincer HH, Coleman SA, Hopkins KP. Observations on the clinical characteristics of oral lesions showing histologic epithelial dysplasia. Oral Surg Oral Med Oral Pathol 1972; 33:389-99.
89. Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer 1984; 53:563-8.
90. Vedtofte P, Holmstrup P, Hjorting-Hansen E, Pindborg JJ. Surgical treatment of premalignant lesions of the oral mucosa. Int J Oral Maxillofac Surg 1987; 16:656-64.
91. Roodenburg JL, Panders AK, Vermey A. Carbon dioxide laser surgery of oral leukoplakia. Oral Surg Oral Med Oral Pathol 1991; 71:670-4.
92. Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, and others. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med 1986; 315:1501-05.
93. Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, and others. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med 1993; 328:15-20.
94. Toma S, Benso S, Albanese E, Palumbo R, Cantoni E, Nicolo G, and others. Treatment with oral leukoplakia with beta- carotene. Oncology 1992; 49:77-81.
95. Epstein JB, Wong FL, Millner A, Le ND. Topical bleomycin treatment of oral leukoplakia: a randomized double-blind clinical trial. Head Neck 1994; 16:539-44.
96. Decroix Y, Ghossein NA. Experience of the Curie Institute in treatment of cancer of the mobile tongue: I. Treatment policies and results. Cancer 1981; 47:496-502.
97. Callery CD, Spiro RH, Strong EW. Changing trends in the management of squamous carcinoma of the tongue. Am J Surg 1984; 148:449-54.
98. Mendenhall WM, Parsons JT, Stinger SP, Cassisi NJ, Million RR. T2 oral tongue carcinoma treated with radiotherapy: analysis of local control and complications. Radiother Oncol 1989; 16:275-81.
99. Nason RW, Sako K, Beecroft WA, Razack MS, Bakamjian VY, Shedd DP. Surgical management of squamous cell carcinoma of the floor of the mouth. Am J Surg 1989; 158:292-6.
100. Wildt J, Bjerrum P, Elbrond O. Squamous cell carcinoma of the oral cavity: a retrospective analysis of treatment and prognosis. Clin Otolaryngol 1989; 14:107-13.
101. Soderholm AL. Oral carcinoma of the mandibular region. Br J Oral Maxillofac Surg 1990; 28:383-9.
102. Franceshi D, Gupta R, Spiro RH, Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg 1993; 166:360-5.
103. Kraus DH, Vastola P, Huvos AG, Spiro RH. Surgical management of squamous cell carcinoma of the base of the tongue. Am J Surg 1993; 166:384-8.
104. Lefebvre JL, Coche-Dequeant B, Buisset E, Mirabel X, Van JT, Prevost B. Management of early oral cavity cancer. Experience of Centre Oscar Lambret. Eur J Cancer B Oral Oncol 1994; 30B:216-20.
105. Haggerty JL. Early detection and counseling of problem drinking. In: The Canadian guide to clinical practice health care. The Canadian Task Force on the Periodic Health Examination. Ottawa: Minister of Supply and Services, 1994.
106. DiGuiseppi C, Atkins D, Woolf SH (eds.). US Preventive Services Task Force. Guide to clinical preventive services, 2nd edition. Alexandria, VA: International Medical Publishing, 1996.
107. United Kingdom working group on screening for oral cancer and precancer. Conclusions and recommendations. Community Dent Health 1993; 10(Suppl 1):87-9.
108. Rodrigues VC, Moss SM, Tuomainen H. Oral cancer in the UK: to screen or not to screen. Oral Oncol 1998; 34:454-65.
109. American Cancer Society. Update January 1992: the American Cancer Society guidelines for the cancer-related checkup. CA Cancer J Clin 1992; 42:44-5.
110. Doherty SA. Basic issues in screening for oral cancer among male subpopulation. J Tenn Dent Assoc 1989; 69:26-9.
111. Meskin LH. Views: oral cancer — the forgotten disease. J Am Dent Assoc 1994; 125:1042-5.
112. O’Malley MS, Fletcher SW. US Preventive Services Task Force. Screening for breast cancer with breast self-examination. A critical review. JAMA 1987; 257:2196-203.
113. Speight PM, Elliot AE, Jullien JA, Downer MC, Zakzrewska JM. The use of artificial intelligence to identify people at risk of oral cancer and precancer. Br Dent J 1995; 179:382-7.
114. Downer MC, Jullien JA, Speight PM. An interim determination of health gain from oral cancer and precancer screening: 3. preselecting high risk individuals. Community Dent Health 1998; 15:72-6.
115. Amsel Z, Strawitz JG, Engstrom PF. The dentist as a referral source of first episode head and neck cancer patients. J Am Dent Assoc 1983; 106:195-7.
116. Paauw DS, Wenrich MD, Curtis JR, Carline JD, Ramsey PG. Ability of primary care physicians to recognize physical findings associated with HIV infection. JAMA 1995; 274:1380-2.
117. Lovas JL, Daley TD, Kaugars GE, Wright JM. Errors in the diagnosis of oral malignancies. J Can Dent Assoc 1993; 59:935-8.
118. Maguire BT, Roberts EE. Dentists’ examination of the oral mucosa to detect oral cancer. J Public Health Dent 1994; 54:115.
119. Yellowitz JA, Goodman HS, Wertheimer DA. Physicians’ and dentists’ oral cancer knowledge and practices. J Public Health Dent 1994; 54:115.