• Jim Grisdale, BA, DDS, Dip. Pros., Dip. Perio., MRCD(C) •
© J Can Dent Assoc 1999; 65:559-62
[Ridge Preservation|Use of Barrier Membranes|Case Review |Conculsion |References]
Prevention of alveolar bone loss and maintenance of alveolar bone structure are mandatory for long-term stability of conventional or implant-supported prosthetic appliances. Extraction of teeth may result in 40% to 60% alveolar bone loss in a period of two to three years.1,2 Several conditions, including aging, facial lines, unesthetic dental restorations and loss of vertical dimension, are often accelerated by premature loss of facial bone. When the jawbone no longer holds teeth, the alveolar bone recedes, resorbs and disappears.1 Bone maintenance is the procedure of preserving bone after tooth loss.3 Every attempt should be made at the time of tooth loss to preserve the height and width of the jawbone.
An unsatisfactory ridge morphology may result from periodontal disease, trauma or endodontic complications. Consideration must be given, during tooth extraction, to the final shape of the alveolar ridge and overlying soft tissue and to the optimal esthetic and functional result of the final tooth replacement. Soft and hard tissue complications can lead to unsatisfactory results for the patient, including unacceptable tooth morphology, poor phonetics and lack of confidence in denture stability. These problems may be prevented at the diagnosis and consultation stage. Contemporary ridge preservation techniques provide the dentist with the means to deliver the required cosmetic, phonetic and functional requirements of the most demanding case.
Bone autografts and allografts are widely used by orthopedic surgeons due to increased demand for skeletal reconstruction.4 Dentists also use autografts, allografts and alloplastic materials in the oral cavity, which change the way we view and treat patients. Coupled with osseointegration, ridge reconstruction and implant placement can provide support for a variety of prostheses.5
Routine dental extractions imply open, bleeding wounds where, in many cases, the hope is that a blood clot will form to permit healing by secondary intention. Disruption of the blood clot may cause slow, painful healing. Occasionally, the facial and lingual cortical plates are compressed in an attempt to reduce the width of the wound site. A slight variation in this technique can provide more predictable results and maintain bone height and ridge contour.
The practitioner must be familiar with the advantages and disadvantages of each graft or implant material. Autogenous graft material is safe and does not cause immunogenicity or cross-contamination. There are cases, however, where there is insufficient autogenous bone or where an additional surgical site is contraindicated. Allograft materials such as decalcified freeze-dried bone, which is widely available and has osteogenic potential, may then be used. However, concerns have been expressed regarding a slight risk of immunogenicity and pathogenicity.8 Mellonig and others9 demonstrated that processing demineralized freeze-dried bone allografts results in inactivation of HIV.
More recently, considerable attention has been focused on synthetic materials. Any successful synthesized alternative to autografts and allografts must possess clinically appealing properties and afford the highest potential for restoring lost form and function. An alloplastic material must therefore be: biocompatible with host tissues (i.e., non-toxic, non-allergenic, non-carcinogenic and non-inflammatory), able to stimulate bone induction, resorbable following replacement by bone, radiopaque, capable of withstanding sterilization procedures without compromising desired characteristics, easy to obtain, inexpensive, and stable with respect to variations in temperature and humidity. It must also have sufficient porosity to allow bone conduction and growth into and around the implant and possess physical properties similar to the tissue it replaces.10,11
Because of its potential application to a number of clinical situations and its adaptability to solving long-term problems, synthetic bone is a welcome addition to the array of materials available to the clinician.12 In attempting to provide an ideal bone substitute, clinicians have experimented — with varying degrees of success — with several materials including autografts,13 allografts14 and various apatite compounds.15 Several undesirable characteristics have been associated with these materials, including resorption following successful placement, difficulty in manipulation, brittleness, non-osteogenic properties, and insufficient microporosity to permit integration with regenerating bone. These materials can also be difficult to obtain.
The reconstruction of atrophic maxilla and mandibular ridges presents oral surgeons, implant dentists and prosthodontists with extremely challenging tasks. Extensive surgery, long post-operative recovery and unpredictable results are typically associated with recovering or regaining lost tissue and bone. Ridge preservation needs to be introduced as a modality to avoid the events that follow tooth loss.16 Alveolar bone has a very high turnover rate and is extremely active metabolically. The primary functions of alveolar bone are to support teeth and to respond to high impact stress. If the functions are compromised, alveolar bone loss can be quite rapid and dramatic.17,18 Several theories exist as to why these events occur: atrophy, decreased blood supply, overlying pressure on edentulous ridges from prostheses, and localized inflammation of the bone. During the life of the patient, this process will continue and will result in hard and soft tissue loss.
The concept of bone preservation is perhaps more important now with the greater acceptance of dental implants associated with fixed or semi-fixed prostheses. The clinician needs to preserve both the height and width of the ridge at the time of surgery. The choice of materials to maintain shape and enhance bone fill is an important factor in grafting ridges and sockets. Some of the materials advocated for ridge preservation have shown problems, including exposure, handling difficulties and unpredictability.19 Dense hydroxy apatite particles and root forms, both of which have demonstrated migration and exfoliation, are associated with failures to achieve predictable results. Composites of biocompatible plastics have been used in medicine over the past three decades with proven success in orthopedic, plastic, reconstructive and ophthalmologic surgery, as well as neurosurgery.20
Hard tissue replacement (HTR) polymer (U.S. Surgical Corp., Norwalk, CT) was introduced in dentistry in 1968 in its porous molded form,21,22 and in the mid-70s as a particulate porous material. The earliest clinical trials were performed by clinicians in their own practices, and according to the first statistical analysis report by periodontists, oral surgeons and general dentists, use of HTR polymer demonstrated a 97.9% success rate.23 HTR polymer is a microporous synthetic bone grafting material that combines a polymethylmethacrylate core with a polyhydroxyethyl methacrylate surface, resulting in a biocompatible composite resin.3 This compound interfaces with bone at its outermost calcium graft layers. These polymers have been used in various forms to make contact lenses, prosthetic heart valves, femoral head prostheses, orthopedic bone cement, spinal fusion procedures, and implant devices for sustained release of medications.24 HTR polymer does not produce an inflammatory or immune response after prolonged contact with bone or soft tissue.25 A negative 10 mV surface charge is characteristic of HTR polymer that is compatible with strong bone conductive properties. Additional properties include hydrophilicity, extensive porosity with a 150 to 350µm interbead pore size and a 200µm intrabead pore size, and substantial compressive strength (50,000 psi in particulate form and 5,000 psi in molded form) despite its porous nature.20 In the particulate form, HTR has been effective in repair of periodontal defects and other bony defects (cysts, tumors, granulomas),26,27 in augmentation of the edentulous ridge, for placement around implants, and in association with immediate tooth-root replacement after extraction.
A significant loss of alveolar bone following single or multiple extractions can be prevented with the use of HTR polymer material.20 Adjacent teeth may be protected from proximal bone loss following extraction for cosmetic, periodontal and functional reasons, and may prevent facial collapse and bite collapse. Ridge augmentation can be classified as an immediate or delayed procedure. Immediate use of HTR polymer can maintain and increase the height and width of the alveolar ridge for future restorative or prosthetic replacement. Delayed augmentation can help to correct ridge atrophy as a consequence of earlier extractions. Porous particulate or molded porous forms of HTR polymer may be considered to increase the dimensions of the alveolar ridge and to correct bony defects.
Bone graft material may benefit from the placement of a barrier membrane to help decrease fibrous tissue in-growth, prevent loss of the material and protect the underlying tissue from bacterial invasions. Both resorbable and non-resorbable materials have been successful in this capacity. Collagen-derived materials, even when used without primary closure, appear to quickly adhere and incorporate into adjacent tissues. Since most manufacturers advocate primary closure with polylactic and polyglycolic acid membranes, more time is required to accomplish the intended purpose of protecting the graft material. Non-resorbable materials such as high-density polytetrafluoroethylene (PTFE), available in varying shapes and textures, may also be used. These materials may be exposed to the oral environment and are typically removed after three weeks.
Certain criteria should be considered in selecting a membrane, including ease of use, use without primary closure possible, biocompatibility and tolerance by the tissues, sufficient stability for the bone graft material to consolidate, and cost. Following single tooth extraction, the use of a collagen-derived (xenograft) material appears to function well as a barrier and meets the above criteria. In the case of multiple extractions, a membrane barrier may not be required with HTR polymer material when primary closure is effected. However, if secondary intention healing is anticipated, a non-resorbable material is often chosen.
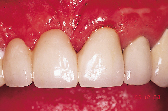
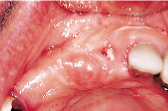
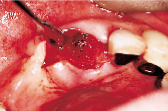
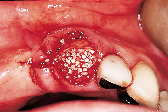
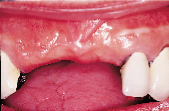
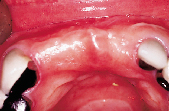
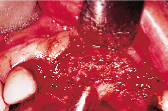
Figures 1 to 10 illustrate the use of a synthetic grafting material, HTR-24 (Septodont of Canada Inc., Cambridge, Ont.) and Hemocollagene (Septodont of Canada Inc., Cambridge, Ont.) in a 68-year-old patient. The patient’s medical history includes hypertension, for which she is medicated on a daily basis. Tooth 21, an abutment for a fixed bridge replacing teeth 11 and 12, had a fractured root that necessitated the sectioning of pontic teeth 11 and 12 and the extraction of tooth 21. A ridge preservation/augmentation procedure was done when tooth 21 was extracted. Approximately 12 months later, two Endopore implants (Innova Corp., Toronto, Ont.) were placed in the approximate positions of teeth 12 and 22. Healing has been uneventful and the patient will proceed in the near future with permanent restoration to replace a pre-existing fixed bridge.
 |
 |
 |
 |
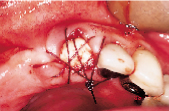 |
| Figure 1: Pre-operative, failed abutment tooth no. 21, recurrent caries. | Figure 2: Fixed bridge removal exposing no. 21 root. | Figure 3: No. 21 root extracted preserving maximum bone possible. | Figure 4: Placement of HTR-24. | Figure 5: Placement of Hemocollagene and 4-0 Vicryl suturing. |
 |
 |
 |
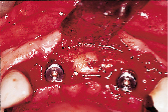 |
| Figure 6: Post-surgical result, 10 months later, facial view. | Figure 7: Post-surgical result, ten months later, incisal view. | Figure 8: Surgical exposure of augmented ridge. | Figure 9: Placement of two Endopore implants. |
 |
| Figure 10: Radiograph of no. 21 and 12 after HTR and Endopore implants. |
Ridge maintenance and replacement are procedures that appear to be gaining in popularity as modalities to facilitate and provide more predictable tooth replacement by restorative and prosthetic means. HTR polymer is well accepted, biocompatible, easy to use and does not cause an apparent inflammatory response. Post-surgical bleeding does not appear to be a problem due to the hydrophilic nature of this material. HTR material can be aggregated with blood and saline, and may be used with antibiotics directly in preparation of the material. Since approximately 10% of HTR polymer is non-resorbable, osseous fill can readily be seen and monitored radiographically during healing. This calcified, microporous copolymer material is a welcome addition to the armamentarium of the dental practitioner.
Dr. Grisdale is in private practice in Vancouver, B.C.
Reprint requests to: Dr. Jim Grisdale, Suite 805 - 805 West Broadway, Vancouver, BC V5Z 1K1
The author has no declared financial interest in any company manufacturing the types of products mentioned in this article.
1. Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: a mixed longitudinal study covering 25 years. J Prosthet Dent 1972;27:120-32.
2. Atwood DA. Some clinical factors related to the rate of resorption of residual ridges. J Prosthet Dent 1962; 12:441-50.
3. Ashman A, Bruins P. A new immediate hard tissue replacement (HTR) for bone in the oral cavity. J Oral Implantol 1982; 10:419-52.
4. de Boer HH. The history of bone grafts. Clin Orthop 1988; 226:292-8.
5. Tolman, DE. Advanced residual ridge resorption: surgical management. Int J Prosthodont 1993; 6:118-25.
6. Rissolo AR, Bennett J. Bone grafting and its essential role in implant dentistry. Dent Clin North Am 1998; 42:91-116.
7. Lekovic V, Kenney EB, Weinlaender M, Han T, Klokkevold P, Nedic M, and others. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontal 1997; 68:563-70.
8. Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop 1989; 240:129-36.
9. Mellonig JT, Prewett AB, Moyer MP. HIV inactivation in a bone allograft. J Periodontal 1992; 63:979-83.
10. Bissada NF, Hangorsky U. Alveolar bone induction: alloplasts. Dent Clin North Am 1980; 24:739-49.
11. Ganeles J, Listgarten MA, Evian CI. Ultrastructure of durapatite — periodontal tissue interface in human intrabony defects. J Periodontal 1986; 57:133-40.
12. Ashman A. On implantable materials. N Y J Dent 1974; 44:180-5.
13. Dragoo MR, Sullivan MC. A clinical and histologic evaluation of autogenous iliac bone grafts in humans: Part I. Wound healing 2 to 8 months. J Periodontal 1973; 44:599-613.
14. Hodosh M, Shklar G, Povar M. Current status of the polymer tooth implant concept. Dent Clin North Am 1970; 14:103-15.
15. Krejci CB, Bissada NF, Farah C, Greenwell H. Clinical evaluation of porous and nonporous hydroxyapatite in the treatment of human periodontal defects. J Periodontal 1987; 58:521-8.
16. Cranin AN, Simons A, Klein M, Kirakian A, Ashman A, Colmery BH 3rd. The use of a particulate, microporous, calcified copolymer as a ridge maintenance device in dogs. J Vet Dent 1995; 12:53-8.
17. Denissen HW, Kalk W, Veldhuis HA, van Waas MA. Anatomic consideration for preventive implantation. Int J Oral Maxillofac Implants 1993; 8:191-6.
18. Lam RV. Effect of root implants on resorption of residual ridges. J Prosthet Dent 1972; 27:311-3.
19. Ashman A. Use of polymeric, ceramic and other materials as synthetic bone in implantology. Endosteol Dental Implants. McKinney Ed. Mosby Chap 1991; 34:427-37.
20. Ashman A, Bruins P. Prevention of alveolar bone loss postextraction with HTR grafting material. Oral Surg Oral Med Oral Pathol 1985; 60:146-53.
21. Ashman A. Acrylic resin tooth implant — a case report. J Oral Implantol 1970; 1:117-9.
22. Ashman A. Acrylic resin tooth implant: a progress report. N Y J Dent 1971; 41:65-8.
23. Norman BA. HTR polymer bone-grafting material: a clinical survey of 647 cases. Compendium 1987; 8:821-2.
24. Boyne PJ, Sheer PM. Bone inductive effect of skeletal growth factor with HA and HTR polymer in synthetic matrices. Abstracts of papers presented at the American Association of Oral and Maxillofacial Surgeons meeting, September, 1986.
25. Ashman A, Moss LM. Implantation of porous polymethylmethacrylate resin for tooth and bone replacement. J Prosthet Dent 1977; 37:657-65.
26. Murray VK. Polymer implantation in periodontic endodontic lesions. Two case reports. N Y J Dent 1991; 57:25-7.
27. Yukna RA. HTR polymer grafts in human periodontal osseous defects. I. 6-month clinical results. J Periodontal 1990; 61:633-42.