Bond Strength of Acrylic Resin to Cobalt-Chromium Alloy Treated with the Silicoater MD and Kevloc Systems
Simona Pesun, B.Sc., DMD
Randall D. Mazurat, B.Sc., DDS
ABSTRACT
Background
An optimum bond strength at the resin-metal interface of a prosthesis is essential to the success of that prosthesis. Silicoater MD and Kevloc are two systems used to increase bond strength at the resin-metal interface. This study investigated the effect that surface pretreatment and chemical shelf life had on the bond strength of silicoated samples and compared the shear bond strength of the two systems.
Methods
Test samples consisted of autocure denture resin attached to cylinders of chrome cobalt that had been treated either with the Silicoater MD or the Kevloc bonding system. The shear strength of the resin-metal interface was tested using a universal testing machine. The following variables were tested: various silicoating pretreatments; shelf-life period of the silanating chemicals; the method of silicoating. The shear strength of repairs using the Kevloc system were also tested.
Results
Surface pretreatment using aluminium oxide and sandblasting significantly enhanced the shear bond strength. The shelf life of the chemicals used in the silicoating process did not affect the bond strength over a six-week testing period. Samples treated with the Kevloc system showed significantly higher bond-strength values (17.3 " 1.7 MPa) and less variability than samples treated with the Silicoater MD system (11 " 3.6 MPa). In addition, all the samples repaired with the Kevloc system had bond strengths comparable to the original Kevloc-treated samples.
Conclusion
The Kevloc system has the potential to provide a more optimal resin-metal bonding system, a stronger and more consistent bond and a simpler laboratory technique.
MeSH Key Words:
dental bonding/methods; denture, partial, fixed, resin-bonded; materials testing; silanes.
© J Can Dent Assoc 1998; 64:798-802
This article has been peer reviewed.
[ Methods | Summary of Investigation Components | Results | Mode of Bond Failure | Discussion | Summary and Conclusions ]
Introduction
The bond strength of the resin-metal interface of a prosthesis is a key factor in determining the serviceability of that prosthesis. Separation of the resin from the metal due to a compromised or weak bond can lead to microleakage, discoloration and total separation of the resin from the metal.1,2 Resin-metal bonding systems as an addition to or replacement for conventional mechanical retentive elements aim at reducing the incidence of failure at the resin-metal interface. An optimum resin-metal bonding system would include a simple procedure producing maximum retention and high, consistent bond-strength values.3
Mechanical retentive elements such as latticework, mesh, beads and posts are incorporated into the design of cast frameworks to retain the acrylic resin matrix. Additional design elements such as external and internal finish lines and tissue relief are used to ensure a sufficient bulk of acrylic for strength. Macromechanical retention may compromise the strength of the acrylic matrix by creating areas of strength concentration or by decreasing the thickness of the acrylic resin.4 The potential for separation of the resin from the metal is also influenced by the difference in thermal expansion coefficients of the resin and metal. Reducing or eliminating macromechanical retentive elements could potentially increase the strength of the resin-metal interface.
Micromechanical retentive elements and chemical bonding systems purport to decrease the necessity for macromechanical retention. They offer the advantage of reduced impingement of the metal framework on the resin matrix; with the increased bulk of resin comes increased strength. These elements and systems also have the advantage of a reduced gap at the resin-metal interface and therefore less susceptibility to microleakage.
Micromechanical means of retention include sandblasting, electrochemical etching and chemical etching.5 Chemical bonding includes adhesive cements, tin electroplating, porous metal coating, tribochemical coating and silicoating.6
A discussion of the clinical applications of resin-metal bonding and an in-depth review of the Silicoater MD and Kevloc systems appeared in our previous article.7
This laboratory investigation arose out of clinical observations of failures occurring at the resin-metal interface of prostheses that had undergone chemical bonding using silicoating (Figs. 1, 2). Our intent was to investigate various aspects of the chemical bonding technique in an effort to explain the variance in bond strength we were observing clinically. Our study used the Silicoater MD and Kevloc systems to bond acrylic resin to cobalt chromium alloy.
The study had three aims:
| 1 | to determine the effect that surface pretreatment and chemical shelf life had on shear bond strength of silicoated samples; |
| 2 | to evaluate the differences in bond strength of samples treated with the Silicoater MD and Kevloc systems; |
| 3 | to determine the bond strength of samples that were debonded and subsequently repaired using the Kevloc system. |
Patterns were designed as cylinders 12 mm tall with a 6.5 mm diameter. The patterns were cast in cobalt chromium alloy (Vitallium) by a commercial laboratory (Austenal) and provided to the investigators for testing. The cast patterns were prepared for chemical bonding according to the manufacturer's (Kulzer Heraeus) written instructions for the Silicoater MD and Kevloc systems. Samples were prepared for testing by fitting a #9 hard copper band to the metal patterns, and filling the band to a height of 5 mm with autocure denture resin (Palopress Vario, Kulzer Heraeus). Samples were dry stored for one week, at which time the bands were removed.
All samples were shear tested in a universal testing machine at a constant cross-head speed of 1 " 0.3 mm/min, 0.1 mm from the resin-metal interface (Fig. 3). Sample preparation and testing were conducted by one investigator.
Summary of the Investigation Components
1. Testing of silicoated samples treated with different
sample pretreatments
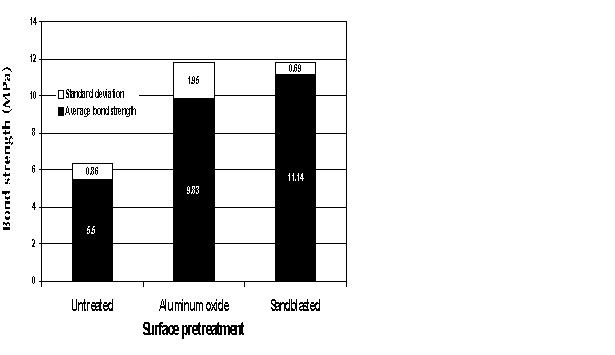 |
| Fig. 4: Bond strengths for different surface pretreatments. |
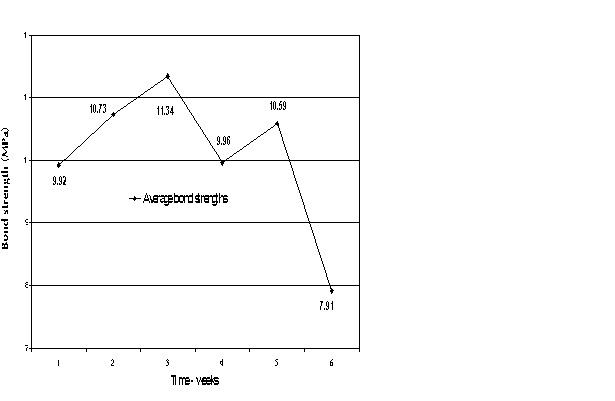 |
| Fig. 5: Mean bond strengths as affected by chemical shelf life. |
Samples were divided into three groups: a control group that received no micromechanical pretreatment and the subject groups that were pretreated either with abrasion with 110-mm aluminium oxide for 30 seconds or with abrasion with 250-mm sand at 5.5 bar for 30 seconds. Twenty-four samples were tested weekly for four weeks, for a total of 96 samples.
2. Testing of silicoated samples over the chemical shelf-life period of the silanating chemicals
Samples in the subject groups that had received micromechanical pretreatment were prepared on a weekly basis and shear tested one week post preparation. The trial period was six weeks. Twelve samples were tested weekly, for a total of 72 samples.
3. Testing of samples prepared using either the Silicoater MD or Kevloc systems
Samples pretreated with 150-mm aluminium oxide were prepared with the Silicoater MD or the Kevloc system and then tested one week later to determine the difference in shear bond strength between the systems. There were 12 samples in each group, for a total of 24 samples.
4. Testing of samples debonded and subsequently repaired using
the Kevloc system
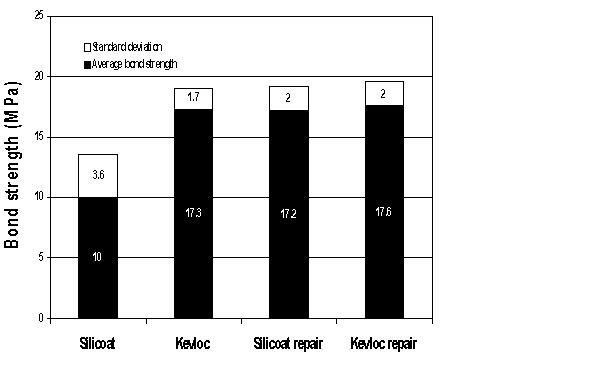 |
| Fig. 6: Mean bond strengths with the Silicoater MD and Kevloc systems. |
The samples tested in part 3 were debonded, repaired using the Kevloc system and then retested for bond strength one week after the repair. There were 10 samples in each group, for a total of 20 samples.
Before conducting the various study components, we conducted a pilot study to determine the sample size of the test groups. The pilot study tested different batches of the silanating chemicals Sililink and Siliseal. The same experimental protocol was used for all components of the study. Results of the pilot study indicated that different batches of the silanating agents did not affect mean bond strength. The silanating agents were evaluated over four weeks. Six samples in each group were tested weekly, for a total of 48 samples.
The mean bond strengths for each test group were calculated and the values were statistically evaluated.
1. Effect of surface pretreatment on bond strength (n = 12)
There was a significant difference in bond strengths between untreated samples and samples pretreated to provide micromechanical retention. The method of providing micromechanical retention _ aluminium oxide or sandblast abrasion _ was not statistically significant according to a multiple analysis of variance (p < 0.05) (Fig. 4).
2. Silicoating as affected by the chemical shelf life
Samples pretreated to produce micromechanical retention were tested over six weeks. The shear bond strength over the period did not correlate to the age of the chemicals (Sililink or Siliseal). The difference in bond values was not statistically significant (Fig. 5).
3. Comparison of samples treated with either the Silicoater MD or Kevloc systems
Student's t-test demonstrated a significant difference between the two systems. The Kevloc treatment produced higher mean bond strengths than the Silicoater treatment (p < 0.01) (Fig. 6).
4. Repair of samples that were debonded and subsequently rebonded using the Kevloc system
The student t-test demonstrated no significant difference (p < 0.05) in bond strength for samples that had been bonded with either system, debonded and then repaired with the Kevloc system. All the repaired samples had a mean bond strength comparable to that of the original Kevloc-treated samples (Fig. 6).
There are four classifications of bond failures:1
1. adhesive failure between the alloy and bonding agent;
2. cohesive failure in the bonding agent;
3. adhesive failure between the bonding agent and resin;
4. cohesive failure in the resin. Visual inspection of photomicrographs and inspection under compound microscope revealed that the mode of failure had adhesive and cohesive elements. The silicoating treatment primarily demonstrated adhesive failure, while the Kevloc-treated samples primarily showed cohesive failure
. ![]() [ Top ]
[ Top ]
As a pilot study we tested different batches of the silanating chemicals used in the Silicoater MD system. Statistically, the chemicals Sililink and Siliseal did not contribute to bond-strength variability among samples. Given the limited sample size and the number of batches tested, it is possible that a clinically significant bond-strength difference might occur due to chemical variance between batches. However, the variance in bond strength values demonstrated in this study was not attributed to any chemical variable, because the same batch of chemicals was used throughout the sample preparation. Similarly, the test procedures and investigator were constant throughout the study, in an attempt to control variation due to procedure or researcher.2
The first variable investigated for the Silicoater MD system was the effect of surface pretreatment. Several previous studies have evaluated this step, with conflicting recommendations on the necessity, method and degree of pretreatment.3,8 The elimination of metal pretreatment would be an asset in both the lab and the clinic. In the lab it would eliminate a time-consuming and technique-sensitive procedure, thereby simplifying the technique. In the clinic it would reduce the potential for inadvertent damage due to alteration of the framework surface. Our results indicate that micromechanical pretreatment is required to produce stronger bonds than are achievable by silicoating alone. The pretreatment methods _ aluminium oxide abrasion and sandblasting _ are recommended in the literature or by the manufacturer. Both pretreatment methods produced similar bond-strength values in this study. The considerable amount of variation in bond-strength values among the samples probably contributed to the lack of distinction between the surface pretreatments.
The second variable investigated was the effect of chemical shelf life on bond strength. The manufacturer stipulates an optimum shelf life of four weeks for Sililink and Siliseal after opening. Prior to this investigation, we did not know whether a significant or gradual decrease in bond strength would occur as the shelf-life limit approached. We also did not know whether the shelf-life estimate was accurate. The relatively short shelf life may be a disadvantage for the laboratory, especially if the Silicoater MD is not used on a regular basis. Our results showed no specific trend nor significant drop in bond strength during the six-week testing period. Bond-strength values were variable with no downward trend that would correlate to the age of the materials. The amount of material provided was insufficient to permit further testing beyond the six weeks. For chemicals subject to shelf-life limitations, using a small amount would help address the cost issue associated with a restricted shelf life.
The third aspect of our study investigated the bond strength of a relatively new bonding system, the Kevloc system. The Kevloc system produced the highest resin-metal bond strength; values were significantly higher than those attained with the Silicoater MD system. Furthermore, bond values for repaired samples of debonded Silicoater- or Kevloc-treated samples were comparable to the original Kevloc-treated samples. Additionally and importantly, the variation in bond-strength values was much smaller among the Kevloc samples than the Silicoater samples.
The mode of bond failure with silicoating appeared to be an adhesive failure at the metal-bonding agent interface. The weak link was the bonding mechanism, not the materials. The mode of failure of the Kevloc samples was primarily cohesive in nature, occurring in the resin or bonding-agent layers. The materials, not the bond, were the weak link. The higher, more consistent bond-strength values achieved with the Kevloc system, as well as the cohesive nature of the failures, indicates that the Kevloc system more closely approximates the ideal resin-metal bonding system. The Kevloc system is also a simpler technique to use, as it has fewer steps and uses chemicals with no shelf-life limitations.
Summary and Conclusions
The purpose of this laboratory investigation was to determine the bond strength of acrylic resin bonded to cobalt-chromium alloy treated with either the Silicoater MD or the Kevloc systems. Our findings were as follows:
1. Surface pretreatment of the alloy to improve micromechanical retention produces significantly higher bond-strength values for silicoated specimens. The manner of surface pretreatment was not statistically significant.
2. Shear bond strength is not affected by aging of the silanating chemicals within the shelf-life period of the chemicals.
3. Compared to the Silicoater MD system, the Kevloc system produces significantly higher bond-strength values with less variation among samples.
4. Repair of debonded specimens using the Kevloc system produces a bond with strength comparable to that of the original Kevloc bond.
The Kevloc system comes closer to achieving the goal of an optimum resin-metal bonding system. The simpler laboratory technique and better results should become the focus of future clinical investigation.
Dr. Pesun is in private practice in Winnipeg.
Dr. Mazurat is assistant professor, Department of Restorative Dentistry, University of Manitoba, Winnipeg.
Reprint requests to: Dr. R. Mazurat, Department of Restorative Dentistry, University of Manitoba, D-235 780 Bannatyne Ave., Winnipeg MB R3E 0W2.
The authors have no declared financial interest in any company manufacturing the types of products mentioned in this article.
References
1. Creugers NHJ, Snoek PA, van't Hof MA, Kayser AF. Clinical performance of resin-bonded bridges: a five year prospective study. Part III: Failure characteristics and survival after rebonding. J Oral Rehabil 1990; 17:179-86.
2. Marinello CP, Kerschbaum T, Heinenberg B, Hinz R, Peters S, Pfeiffer P, and others. Experiences with resin-bonded bridges and splints _ a retrospective study. J Oral Rehabil 1987; 14:251-60.
3. Creugers NHJ, Welle PK, Vrijhoef MMA. Four bonding systems for resin-bonded cast metal prostheses. Dent Mater 1988; 4:85-8.
4. Jacobson TE. The significance of adhesive denture base resin. Int J Prosthodont 1989; 2:163- 72.
5. Hansson O. The Silicoater technique for resin-bonded prostheses: clinical and laboratory procedures. Quintessence Int 1989; 20:85-99.
6. Adept Institute. Metal-resin bonding. Adept Report 1991; 2:25-40.
7. Mazurat R, Pesun S. Resin-metal bonding systems: a review of the silicoating and Kevloc systems. J Can Dent Assoc 1998; 64:503-7.
8. Musil R, Tiller HJ. The adhesion of dental resins to metal surfaces. The Kulzer Silicoater technique. 1st ed. Wehrheim: Kulzer Co. Gmbh, 1984; pp. 9-53.